10+ Orbital Diagram Mg
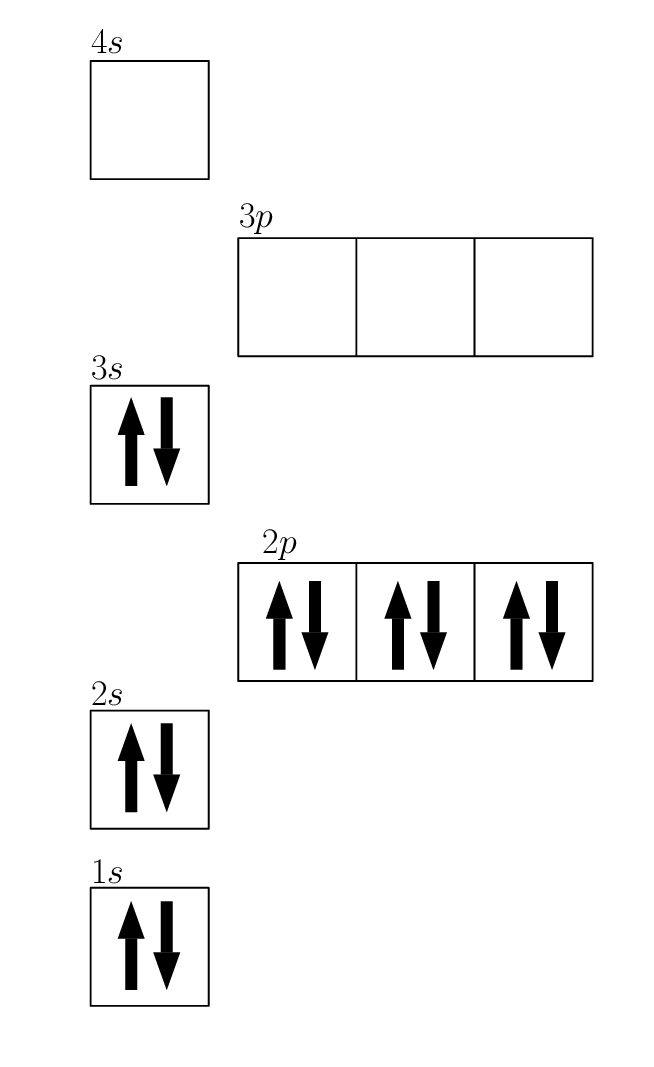
Web Orbital Diagrams Overview Examples. 1s 2 2s 2 2p 6 3s 2.

Shaalaa Com
Therefore the valency of magnesium is 2.

. Web The orbital diagram of Magnesium contains 1s orbital 2s orbital 2p orbital and 3s orbital. Web The four different types of orbitals spd and f have different shapes and one orbital can hold a maximum of two electrons. Thus many students find it confusing that for example the 5 p orbitals fill immediately after the 4 d and immediately before the 6 s.
Web How to Write the Orbital Diagram for Magnesium Mg. To do that we need to find the number of electrons for the Al atom. Orbital is the region of space around the nucleus of an atom where electrons are found.
The p d and f orbitals have different sublevels thus can hold more electrons. Thus many students find it confusing that for example the 5 p orbitals fill immediately after the 4 d and immediately before the 6 s. In this video we will see what orbital diagrams are rules that we follow while determining one for any element and then we will see how to draw one for.
The atomic number of Magnesium is 12. 1s 2 2s 2 2p 6 3s 2 3p 1. The magnesium orbital diagram is a graphical representation of the electron configuration of the magnesium atom.
It explains how to write the orbital diagram notation with arrows of an element. Web Use a qualitative molecular orbital energy-level diagram to predict the electron configuration the bond order and the number of unpaired electrons in S 2 a bright blue gas at high temperatures. Orbital diagram of Lithium Li 4.
Another isoelectronic series is P 3 S 2 Cl Ar K Ca 2 and Sc 3 Ne3 s2 3 p6. Web In order to write the Mg electron configuration we first need to know the number of electrons for the Mg atom there are 12 electrons. As stated the electron configuration of each element is unique to its position on the periodic table.
This diagram shows how the electrons in the magnesium atom are arranged in different orbitals. Orbital diagram of Oxygen O 9. Web Electron configuration of Magnesium Mg Ne 3s 2.
Symbol Mass Number Relative Atomic Mass Isotopic Composition. Web The electron configuration of magnesium shows that it has two unpaired electrons in the last orbital. Orbital diagram of Carbon C 7.
Orbital diagram of Beryllium Be 5. Web Magnesium Mg has 2 electrons in its outer orbit. P Phosphorus What element is represented by this orbital diagram.
Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Orbital diagram of Hydrogen H 2. Web Atoms and ions that have the same electron configuration are said to be isoelectronic.
In this case there are 2 electrons in the 3s orbital which are the valence electrons. Web 72 views 3 months ago Explanation. Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons not to scale.
Web Orbital Diagram of All Elements Diagrams. Study with Quizlet and memorize flashcards containing terms like C Carbon Mg Magnesium S Sulfur and more. The outer orbit refers to the outermost energy level which is the third energy level designated as the 3s orbital in the electron configuration of magnesium.
Web Magnesium Mg has an atomic mass of 12. When we write the configuration well put all 12 electrons in orbitals around the nucleus of the Magnesium atom. Magnesium has a total of 12 electrons and one box can hold up to two electrons.
Orbital diagram of Helium He 3. Each sublevel is labeled by its principal energy level and sublevel. Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons not to scale.
Web In an orbital filling diagram the individual orbitals are shown as circles or squares and orbitals within a sublevel are drawn next to each other horizontally. Electron configuration of Silicon Si. Orbital diagram of Nitrogen N 8.
Web This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. Electron configuration of Aluminum Al Ne 3s 2 3p 1. Orbital Diagram of All Elements Diagrams given Inside Subscribe to our newsletter.
Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Web Na Sodium What element is represented by this orbital diagram. Orbital diagram of Boron B 6.
An orbital diagram or orbital filling diagram is a type of notation which illustrates an atoms electron distribution and electron spin within orbitals. Mg is a chemical element that has the symbol Mg. V Vanadium What element is represented by this orbital diagram.
Examples of isoelectronic species are N 3 O 2 F Ne Na Mg 2 and Al 3 all have the electron configuration 1 s2 2 s2 2 p6. It is a grey shiny solid that bears a close physical resemblance to five other elements of the second column and group 2 alkaline earth metals of the periodic table. Find out about its chemical and physical properties states energy electrons oxidation and more.
Web To write the orbital diagram for the Magnesium atom Mg first we need to write the electron configuration for just Mg. Molecular orbital energy-level diagram bond order and number of unpaired electrons. 1s orbital contains 1 box 2s orbital also contains 1 box 2p orbital contains 3 boxes and 3s orbital contains 1 box.
How many valence electrons does magnesium ionMg 2 have. Web Figure 831 83. Web Magnesium Electron Configuration.
Web Figure 624 Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons not to scale. Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first.
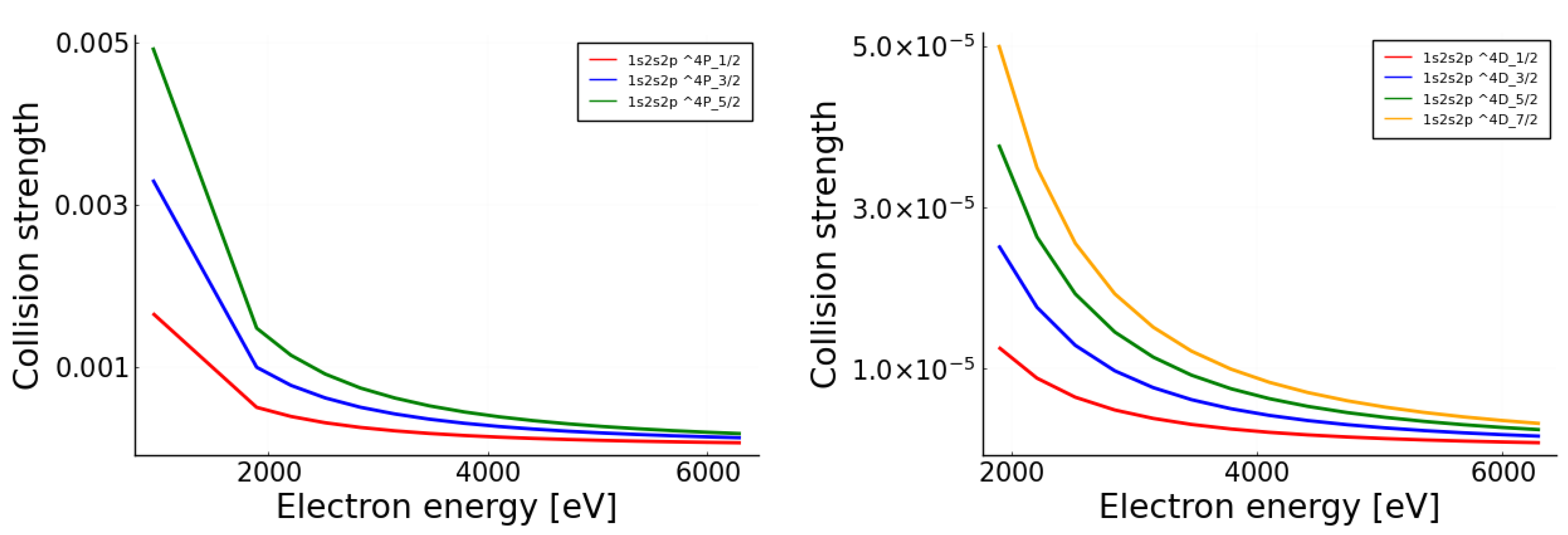
Mdpi

Youtube

Pearson

Acs Publications American Chemical Society

Youtube

1
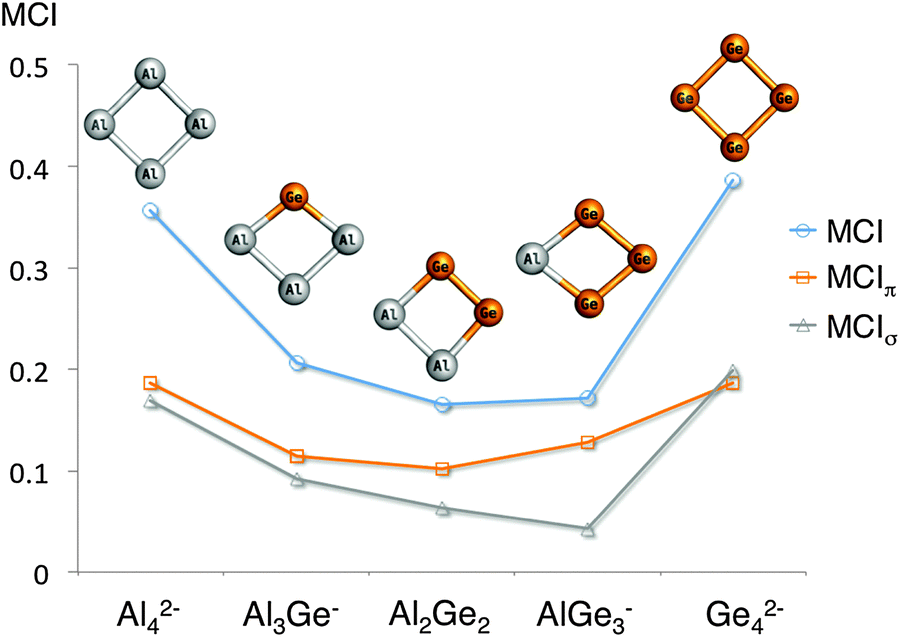
Rsc Publishing The Royal Society Of Chemistry

Slideplayer

Sciencedirect Com
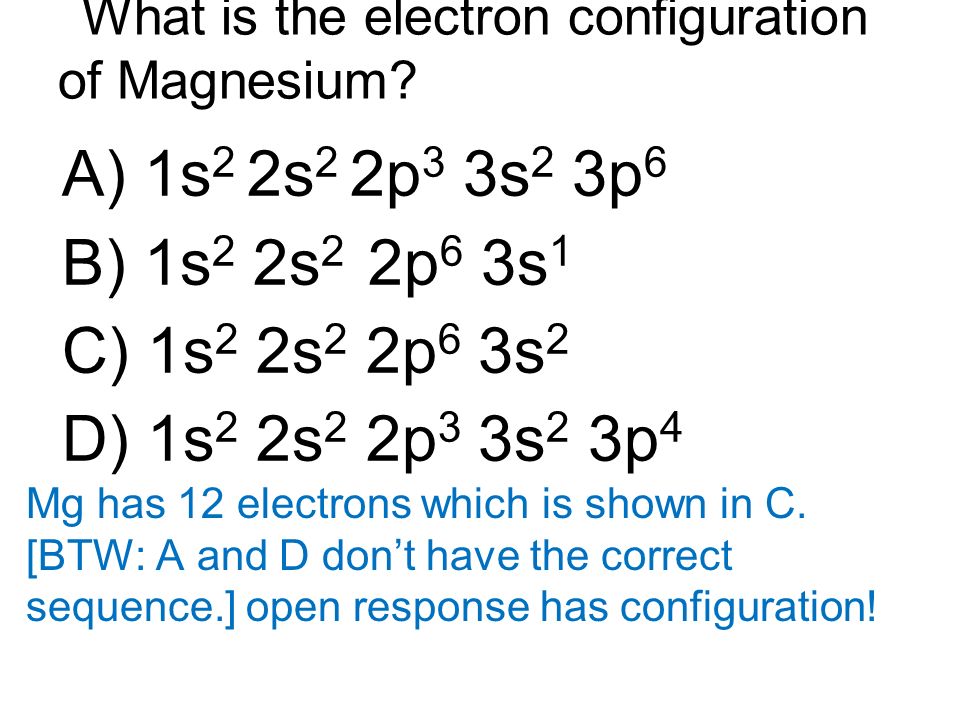
Periodic Table
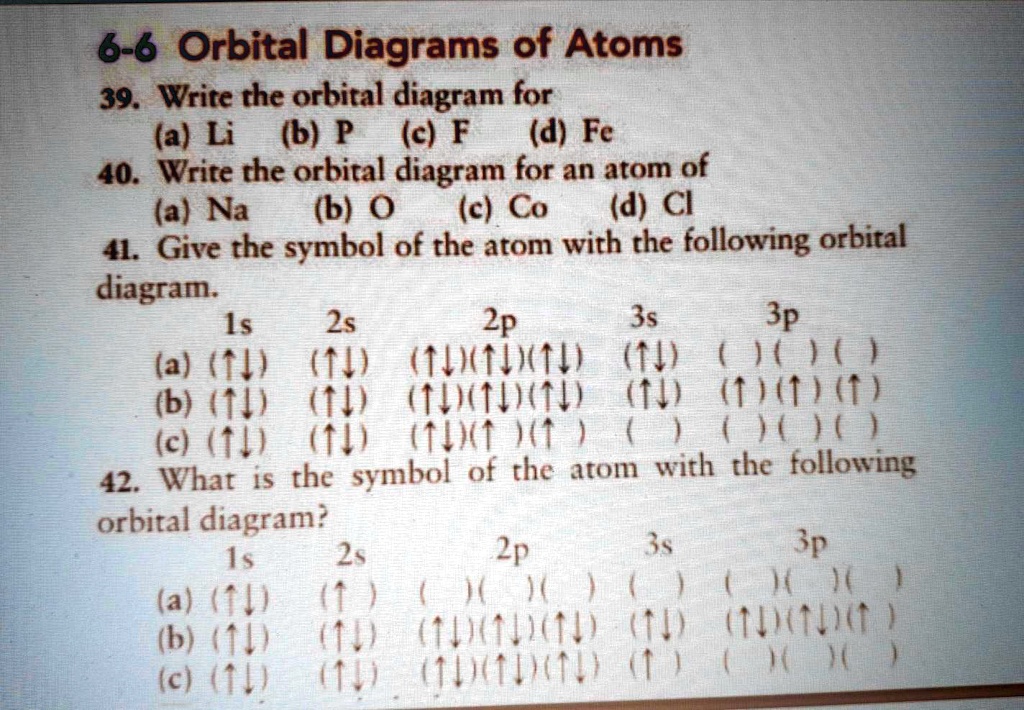
Numerade
Siyavula

Pearson

Pearson

Pearson

Chemistry Europe Wiley

Chemistry Europe Wiley