18+ viral vector services
We strive to exceed our clients expectations on every project while. Viral Vectors Cell Engineering.

Covid 19 Clinical Information The Loop
The acquisition announced this morning sees Germanys CEVEC and its 46 staff join bioprocess giant Cytiva for an undisclosed amount.
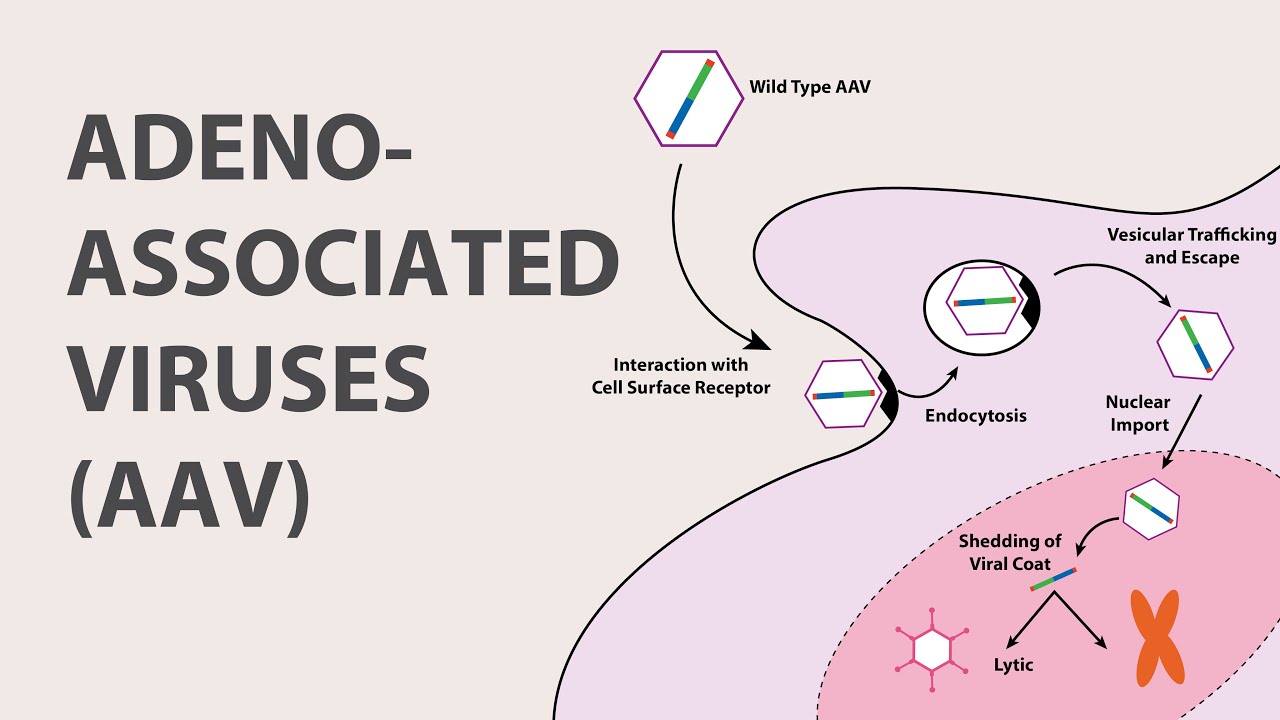
. HEK293 cells are the predominant mammalian platform for viral. These viral vectors are used for preclinical research studies which. To begin custom lentivirus production a suitable mammalian or insect cell line is selected and expanded.
WuXi Biologics utilizes and has experience with a wide variety of viral vectors adenovirus herpes virus vesicular stomatitis virus retrovirus vaccinia virus oncolytic virus for use in the development and GMP manufacturing of viral vaccines both preventative and therapeutic vaccines. The maximum manufacturing capacity using the. Review our services contact our experts today.
Viral vectors are tools commonly used by molecular biologists to deliver genetic material into cellsThis process can be performed inside a living organism or in cell culture Viruses have evolved specialized molecular mechanisms to efficiently transport their genomes inside the cells they infect. Advantages of Cyagens AAV virus packaging service. The Vector Core supports the development and production of viral vectors necessary for gene therapy transfer in research.
The clinical research related virus vector services provided by Creative. Our team of scientific experts will work with you to ensure that you receive AAV lentivirus retrovirus and adenovirus packaging services that. Bioreactor Capacity A Train Capable of up to 500L suspension culture or multi-layer single use vessels for adherent culture.
Flexible network of 45 viral vector drug substance suites worldwide with more than 650000 square feet of cell and gene therapy facilities capacity ensures we can address increasingly. Patheon Viral Vector Services VVS is your end-to-end viral vector CDMO partner from process and analytical development to clinical and commercial supply of viral vectors for your cell or gene therapeutic or vaccine product. We provide high quality consistent viral vector services and are dedicated to the development and production of viral vectors for angiogenesis stem cell regenerative medicine and clinical.
Get to IND Six Months Faster with Patheons Quick to Clinic Viral Vector Services. With more than 14 years of experience in viral vector services Patheon is your end-to-end viral vector CDMO partner from process and analytical development to clinical and. Genezen offers contract process development GMP viral vector production transduced cell manufacturing and testing services.
Quality is at the core of everything we do. June 18 2022 Full-Time Job Description Senior Business Development Executive - Viral Vector Services Massachusetts. T he LakePharma Vector Technology group offers a comprehensive suite of viral vector and cell engineering services which cover vector.
Delivery of genes or other genetic material by a vector is termed transduction and. Development and Production of Viral Vectors. For cell types that are not suitable for lipid-mediated transfection viral vectors are often used.
Research-Grade Viral Vector Packaging Services. For cell types not amenable to lipid-mediated transfection viral vectors are often employed. Explore CBMs viral vector manufacturing capabilities that include Adenovirus AAV Lentivirus HSV other vectors.
Virus-mediated transfection also known as transduction offers a means to reach. CGMP Viral Vector Manufacturing. The viral vector core facility at Sanford Burnham Prebys develops state-of-the-art viral vector-based gene delivery technology.
Covid 19 Vaccines Region Of Durham. Capabilities also include plasmid vector construction and engineering and. Viral Vector Services Massachusetts The.
CGMP Cell. Esco Asters state-of-the-art GMP facility provides the services of viral vector manufacturing for clinical materials and commercial products. The deal brings Cytiva a range of cell.
Our focus is providing unwavering support to overcome. The development of an efficient viral vector manufacturing process is critical in order to ensure that the large amount of material needed for clinical trials is produced efficiently safely and. Its portfolio ranges from lentivirus retrovirus adenovirus AAV.
We have over 20 years experience and expertise in producing adenoviral vectors and have worked with various host cells and support both adherent and suspension. The Vector Core manufactures research grade AAV vectors for both academic institutes and biopharmaceutical clients.

Covid 19 Vaccines Centre For Effective Practice Digital Tools

Phdmc Public Health Dayton Montgomery County
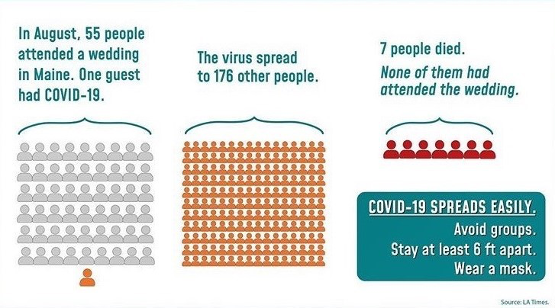
Latest Health Updates Student Health Services At Pcc Student Health Services Pasadena City College

Viral Vector Therapies At Scale Today S Challenges And Future Opportunities Mckinsey

A Comprehensive Review Of The Global Efforts On Covid 19 Vaccine Development Acs Central Science

Innovation In Viral Vector Gene Therapy Unlocking The Promise Mckinsey
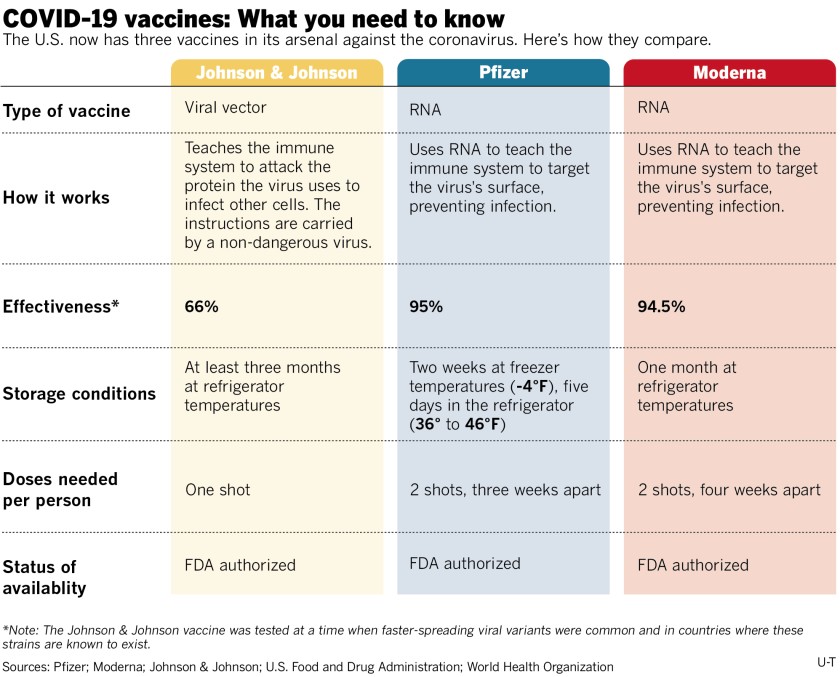
Covid 19 Vaccine Faqs Singing River Health System

How To Go Viral On Tiktok 7 Tips From Brands That Did It Sprout Social

Thermo Fisher Expands Viral Vector Production Capabilities
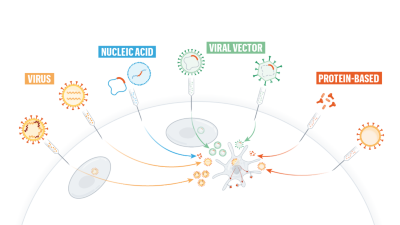
Why Oxford S Positive Covid Vaccine Results Are Puzzling Scientists

Covid 19 Vaccines Region Of Durham

Viral Vector Therapies At Scale Today S Challenges And Future Opportunities Mckinsey

Health And Human Services Utah National Guard Novel Coronavirus 2019
Covid 19 Vaccines Centre For Effective Practice Digital Tools

Department Of Health Communicable Disease Service Covid 19 Vaccination

Covid 19 Vaccine La Dept Of Health
Usda Open Data Catalog Usda